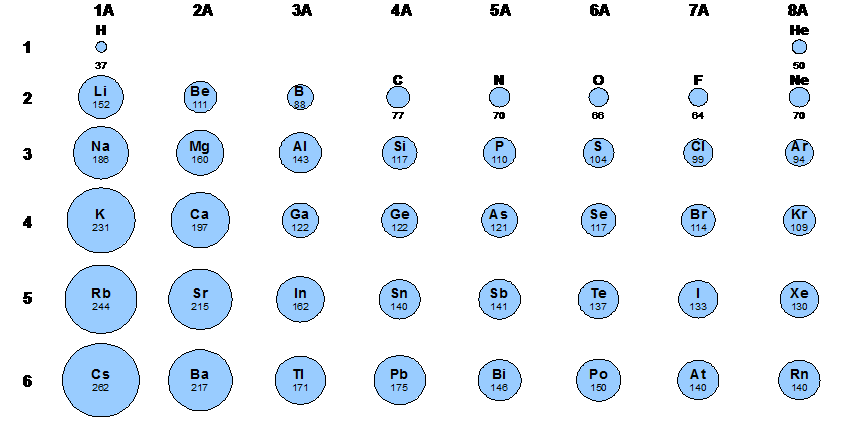
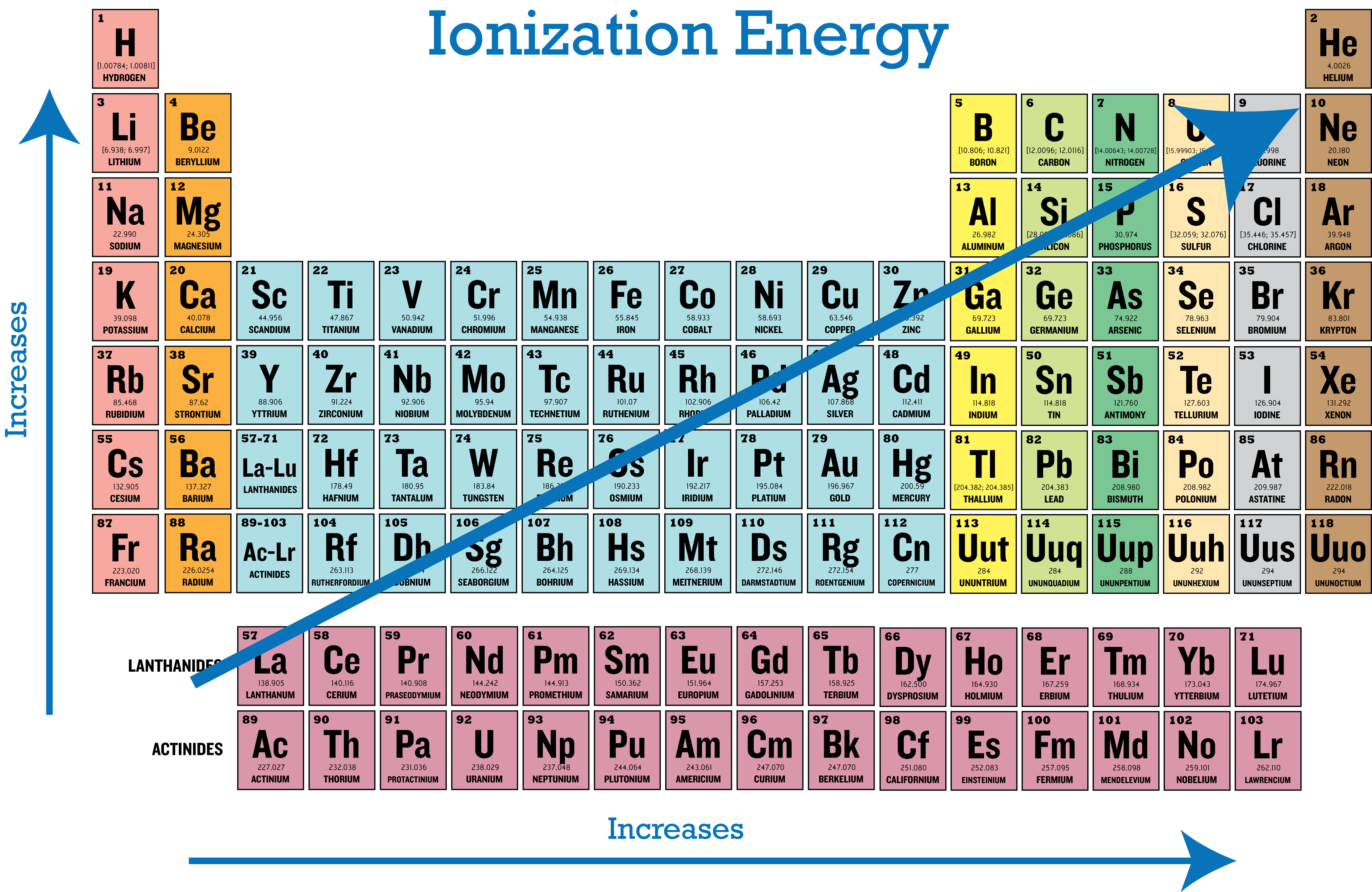
The next periodic trend we learned was over ionization energy. Ionization energy is the energy needed to remove an electron from a gaseous atom. When this electron is removed from the gaseous ion it results in the formation of a cation. Ionization energy occurs each time an electron is removed, meaning their are multiple sets of ionization energies. For this trend, the ionization energy increases as you move up and to the right across the periodic table.
Electron affinity was the next periodic trend we went over in class. Electron affinity is known as the ease with which an electron may be added to an atom, forming an anion. Since some atoms give off energy when an electron is added, some electron affinities are negative. The periodic trend for electron affinity is an increase as you move up and to the right on the periodic table.

The last periodic trend we learned in this lecture was on electronegativity. Electronegativity is the tendency of an atom to draw electrons toward itself when it's chemically combined with another element. An important thing to remember about electronegativity is that there are no units of measurement. The relative trend for electronegativity is an increase as you move up and to the right on the periodic table, excluding noble gases.

http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
Erin, this post really helped me clarify the direction the trends move and what they are.
ReplyDeleteThanks for the pictures, they really helped to simplify all the trends we've learned about.
ReplyDelete