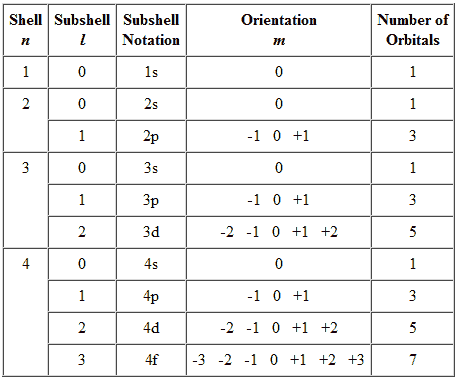
- The first quantum number is the value of the principal energy level, labeled as n
- The second quantum number is the number that determines the type of sublevel, labeled as l
- The third quantum number runs from -1 to +1, labeled as ml
- The fourth, and last, quantum number is spin of the electrons, labeled as ms
No comments:
Post a Comment