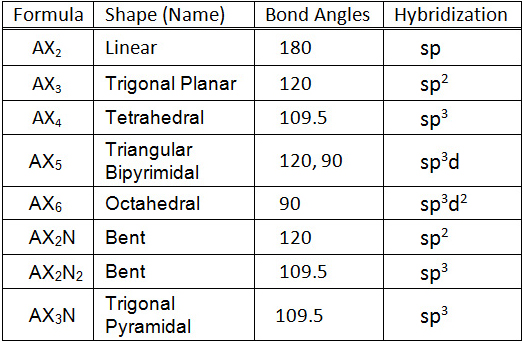
The valence shell electron pair repulsion theory is what is used in order to predict the shape of a molecule. The number of lone pairs and bonded entities present in a molecule will determine it's shape. In particular, we focused on five of the molecular shapes: linear, bent, tetrahedral, trigonal planar, and triganol pyrimidal. A linear molecule has a central atom, consisting of no lone pairs, with two bonded entities. Bent molecules also have two bonded entities but the central atom has two lone pairs. A tetrahedral shaped molecules consists of a central atom with four bonded entities. A molecule without lone pairs on the central atom and three bonded entities is trigonal planar. Lastly, a molecule that is trigonal pyrimidal contains a central atom with two lone pairs and three bonded entities.
We also went over resonance in this lecture. Resonance occurs when a molecule has multiple bonds that can be moved throughout the molecule with the same resulting charges. The picture below shows resonance occurring in an NO
3 molecule. Resonance equalizes both the bond strength and the bond length throughout the molecule.
 Molecular Shapes Practice
resonance practice
Molecular Shapes Practice
resonance practice


No comments:
Post a Comment