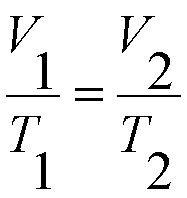In class today we learned about the differences between acids and bases in physical properties and how they were defined by two different scientists, Arrhenius and Bronsted-Lowery.
Physical properties:
Acids Bases
Taste sour Taste bitter
Feel sticky Feel slippery
Turns litmus pink Turns litmus blue
Arrhenius vs. Bronsted-Lowery
Arrhenius
Acids are those species that produce hydrogen ions in solution (H+)
Bases are those species that produce hydroxide ions in solution (OH-)
Bronsted-Lowery
Acids are those species that donate a proton (H+)
Bases are those species that accept a proton (OH-)
We also learned how to determine the conjugate base and conjugate acid in a reaction. By determining the acid and base using either the Arrhenius or Bronted-Lowery definitions, we can then find its conjugate base and conjugate acid. A conjugate acid is the substance that forms when a proton is added to a base. On the other hand, a conjugate base is the remaining substance when a proton is lost from an acid.
This Link has some helpful practice problems for acids bases.