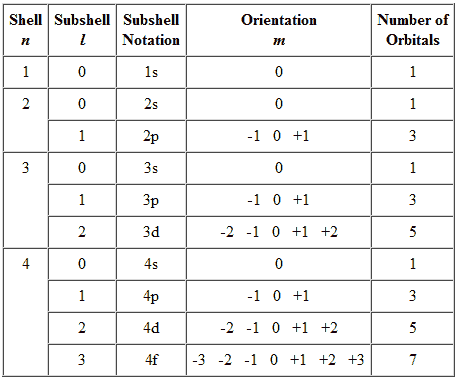
Today's lecture in chemistry was over titrations and equivalence points. A titration is a technique that is used in order to determine the concentration of an unknown acid or base. During the titration, a neutralization reaction, or a reaction that uses equal quantities of acid and base, occurs. A neutralization reaction is reached once the amount of acid and base, with respect to concentrations, are equal and this can be seen when the solution in the Erlenmeyer flask turns a light pink. This change in color is caused by an indicator that is pH sensitive which changes the color once the reaction is complete. An equivalency point in a titration is when the moles that were originally in the solution are equal to the moles after the titration.
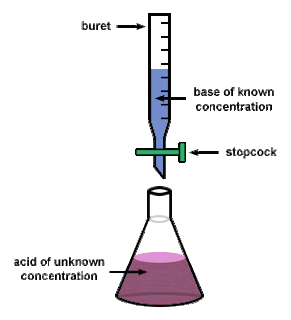
A titration is shown in this picture above. the base is in the buret, being kept there by the stopcock. The Erlenmeyer flask contains the acid which has an unknown concentration, this flask is placed right under the buret. The stopcock is then opened in order to allow the base to go into the acid until the color change occurs.
I found
this site helpful in order to practice some titration calculations