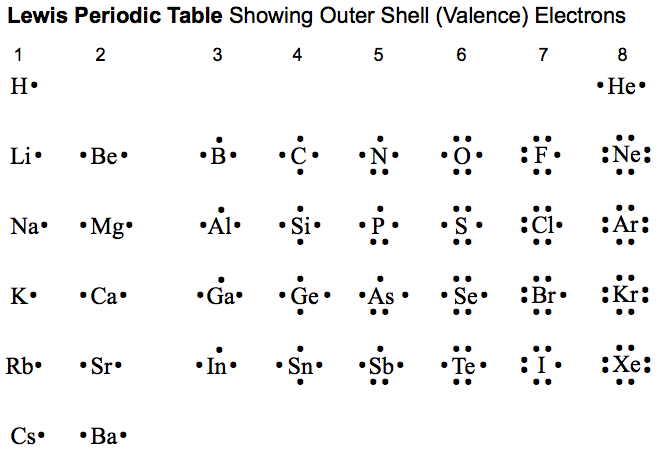
One of the rules we learned regarding electrons in the valence shell was the octet rule. The octet rule states that, with a few exceptions, an atom can not have more than eight electrons in it's outer shell. An example of an exception is beryllium. Beryllium, despite the predicted four valence electron, requires six valence electrons in order to be stable.
Practice
Lewis Dot Structures
No comments:
Post a Comment