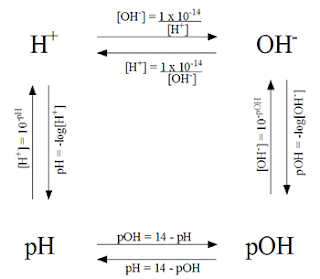
This picture shows how to get from each value to the next in simple problems.
What is the pH of a solution that is 12.5 M HCL?
pH= -log [H+]
= -log(12.5)
= -1.097
In order to solve this problem you have to look at the given information. Since we were given the concentration of Hydrochloric acid, it is the same as being given the concentration of hydrogen ions (acids go with hydrogen, bases go with hydronium). Using the chart, we know that in order to get to the pH with the concentration of hydrogen ions we need to do -log [H+].
Other problems require more than one step in order to get the answer. For example, if you are given the concentration of hydronium ions and asked to find the pH, then you need to find the concentration of hydrogen ions, or the pOH first.
This site helped me practice the conversions from this lecture.
No comments:
Post a Comment